The decision between going with synthetic versus conventional oil is an age-old debate. But what’s the real difference between them when it comes to your engine? Synthetic oil is made up of artificial chemical compounds while conventional is refined crude oil. We’re breaking down the explanations behind why you should pay more for synthetic.
Viscosity Index
In physics, viscosity describes a fluid’s resistance to flow. When an object flows through oil, for example, the oil resists the flowing object and the velocity the object is creating through its motion. Several different factors can affect the flow and resistance such as particles suspended in the liquid. The change in the viscosity due to a temperature change is called the viscosity index (VI).
.png)
Today, we’ll be looking at how the viscosity of the oils changes with temperature. Something with a low VI changes a lot with temperature while something with a high VI has less of a change. The lesser the change, the better.
If we look at the molecules that make up the conventional oil, we notice that these molecules vary in size, shape, and weight compared to the synthetic. This results in a low VI. At low temperatures, the larger molecules resisting the flow cause conventional oil to be thicker. However, due to their varying sizes, smaller molecules exist as well that make conventional oil thinner at higher temperatures.
Synthetic oil molecules are much more uniform which causes a higher VI. This means less change with temperature. So, at low temperatures, you have better flow due to less thickening, and at higher temperatures, you have a better flow due to less oil thinning out. Ultimately an oil that maintains its viscosity at higher temperatures means better wear protection.
Volatility
In chemistry, volatility describes how readily a liquid vaporizes. Some oil can evaporate once it is heated and thus lost. You want as much of that oil to remain a liquid as possible. There is an industry standardized test called the Noack Volatility test that heats oils at 250 degrees Celsius for one hour. Air flows over oils catching any particles being vaporized. After the hour, the liquid’s mass is measured.

With conventional oil, due to its many smaller molecules scattered throughout the liquid, it’s more prone to evaporation and thus losing mass. Plus, what you’re left with is many larger molecules making the oil very thick at lower temperatures. With synthetic, not as much is vaporized, so at lower temperatures, flow remains mostly the same.
So what? This is a quite the afternoon experiment all to buy some crummy oil. Fortunately, this test is on the back of oil containers. Look for ILSAC GF-6 which indicates it passed the volatility test and didn’t lose more than 15% of its mass.
Deposits
Additives can change the VI. To thicken the oil, you use viscosity modifiers. With synthetic oil, you use fewer of them than for conventional oil because your viscosity baseline begins lower. With these additives, you can get your conventional IV to line up equally to synthetic.
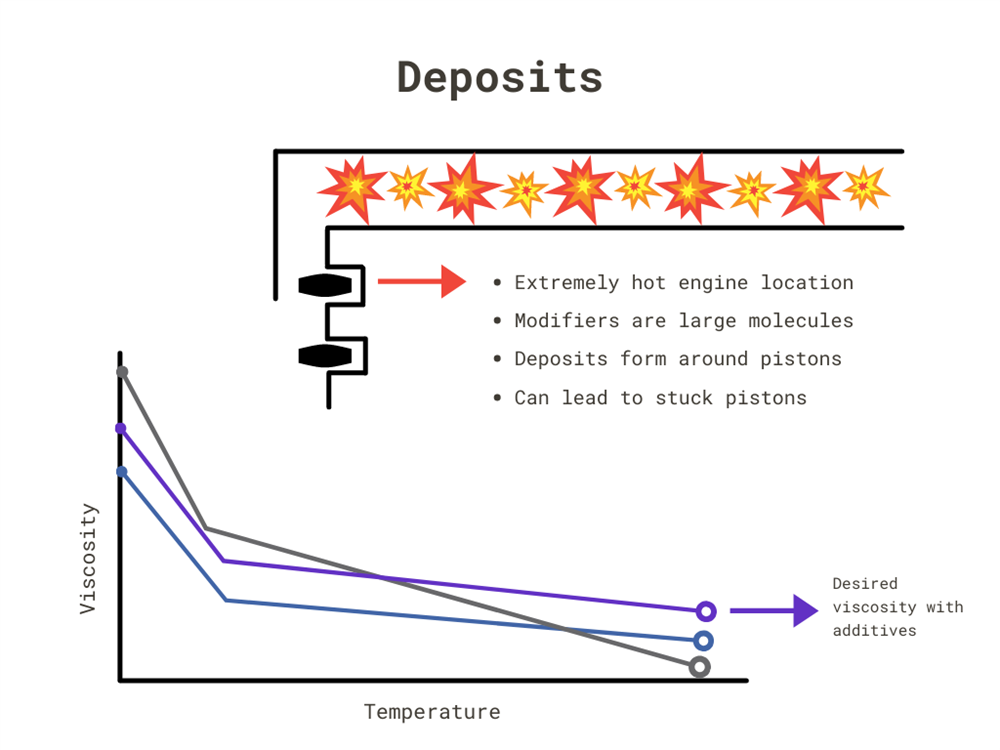
However, viscosity modifiers have very large molecules – 200 to 2,000 times the size of what is considered a large molecule in conventional oil. These are susceptible to creating deposits in the piston rings causing them to lock in place. Locked pistons can cause oil control loss, blowby, and cylinder damage. Because synthetics use fewer additives, they are less susceptible to deposits forming in these high-temperature areas.
Classic car owners can worry less about small, turbo-sized engines, a new trend in the industry that cause more heat in a smaller area. While they may be all rated for the same viscosity, synthetic still can remain thicker at a higher temperature.
Oxidation
A couple of months ago, we talked about how automakers fought corrosion. Rust happens when a metal gives up its electrons to another piece of metal willing to receive it. Typically, a liquid, otherwise known as an electrode, facilitates this transfer. This is also known as oxidation.
Something similar happens with motor oil: oxidation will react with it and change it. This forms a sludge-like substance and makes the molecules heavier. Heat accelerates this oxidation process. When looking at conventional oil, you see more unsaturated molecules.
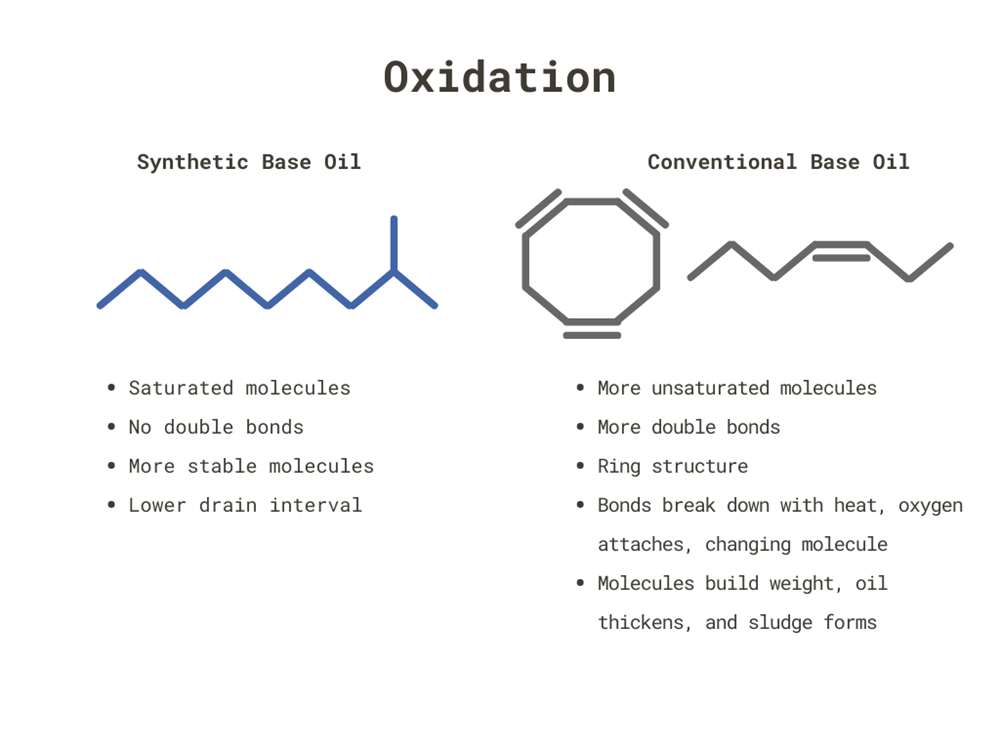
These molecules have ring structures and double bonds that are susceptible to oxygen coming into higher temperatures, attaching to the molecule, and growing them in size. This causes the oil to become thick forcing you to change the oil.
With a synthetic base oil, its saturated molecules do not have ring structures or double bonds which remain stable at high temperatures. Although not impossible, it’s much more difficult for oxygen to get into them. To prolong this process, you either need a stable base oil as with the case of synthetic oil or antioxidant additives that fights the oxidation.
